
Discovery and Research
- Identification of Target Antigens : Focus on identifying and validating antigens relevant to specific cancers and viral infections.
- Technology Development : Innovate and refine minicircle-DNA vaccine technology and advanced CAR T cell designs.
Preclinical Development
- Minicircle-DNA Vaccine Platform : Develop and test vaccine candidates in vitro and in preclinical models to evaluate safety, efficacy, and stability.
- CAR T Cell Engineering : Design and optimize CAR T cells with enhanced targeting capabilities and resistance to tumor microenvironment (TME) challenges.
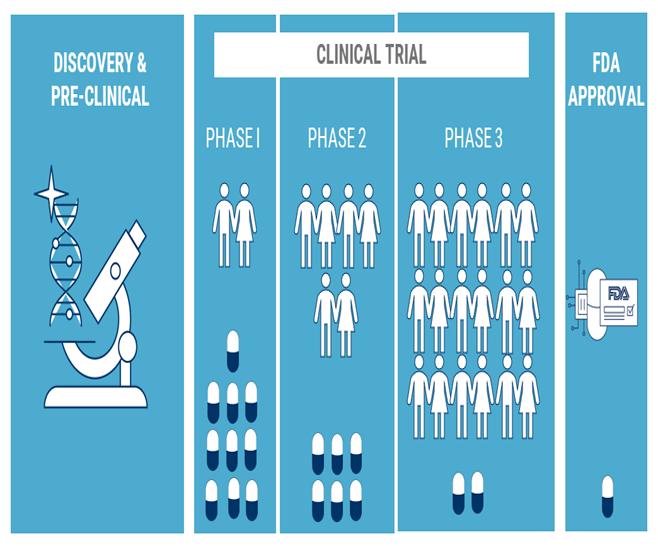
Clinical Development
- Phase 1 Trials : Conduct early-phase clinical trials to assess safety and dosage in healthy volunteers and patients.
- Phase 2 Trials : Evaluate the efficacy of vaccines and CAR T cell therapies in patients with specific cancers or viral infections.
- Phase 3 Trials : Perform large-scale trials to confirm efficacy, monitor side effects, and compare with existing treatments.
Regulatory Approval
- Regulatory Submissions : Prepare and submit documentation for regulatory review to obtain approvals from health authorities.
- Compliance and Quality Assurance : Ensure that all processes meet regulatory standards and guidelines.

Working Together, ideas Come to Life
- Scale-Up Production : Develop and implement scalable manufacturing processes for vaccines and CAR T cells
- Quality Control : Monitor and ensure the quality and consistency of produced therapies.
Market Launch
- Commercialization Strategy : Develop and execute strategies for market entry, including partnerships, distribution, and marketing.
- Patient Access :Facilitate access to therapies for patients through collaborations with healthcare providers and payers.


Post-Market Surveillance
- Ongoing Monitoring :Track the long-term safety and effectiveness of therapies in the broader patient population.
- Continual Improvement :Gather feedback and make iterative improvements based on real-world data and patient outcomes.
